
Aaron DiAntonio, MD, PhD
Alan A and Edith L Wolff Professor, WashU Developmental Biology
- Phone: 314-362-9866
- Email: diantonio@nospam.wustl.edu
Axonal biology in development and disease
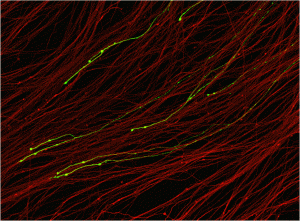
Our laboratory investigates molecular mechanisms that control the structure and function of neural circuits in development and disease. We combine genetic, molecular, neuroanatomical, and electrophysiological studies in both Drosophila and mouse to identify pathways required for the development, maintenance, and regeneration of axons and synapses. Our studies focus on three major areas:
1) Axonal degeneration in disease: Axonal degeneration is a common feature of many neurological diseases including hereditary neuropathies, diabetes, glaucoma, chemotherapy-induced neurotoxicity, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Axonal degeneration is an active process of self-destruction that appears to be naturally primed and waiting for a triggering stimulus that activates the execution phase. We identified the DLK/JNK MAP kinase pathway as the first intrinsic neuronal pathway that promotes axonal degeneration following injury. We are using genome-wide screens in both Drosophila and mice to identify the molecular mechanisms driving axonal degeneration. Identifying and characterizing components of the intrinsic axonal degeneration pathway has identified potential therapeutic targets for the many neurological diseases characterized by axonal degeneration.
2) Axonal regeneration in response to injury: Neuronal repair is greatly impaired by the failure of adult CNS neurons to regenerate axons lost to injury or disease. Remarkably, a prior preconditioning injury can activate an axonal growth program and promote axonal regeneration. We have recently demonstrated that the MAPKKK DLK is a key trigger that induces this preconditioning response. We are investigating the mechanisms by which this regenerative growth program can be activated in order to promote neuronal repair. Finally, we are using high-content automated screening approaches to undertake large-scale drug and RNAi screens in order to identify novel therapeutic candidates that induce axonal regeneration.
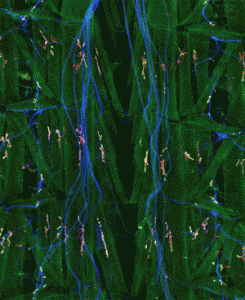
3) Synaptic function: Neurotransmitter is released from the presynaptic cell at specialized sites called active zones. Efficient synaptic transmission requires that active zones contain a normal complement of proteins, and that these specialized release sites be apposed to postsynaptic clusters of neurotransmitter receptor. Little is known of the molecular mechanisms that regulate the protein composition of active zones and ensure the alignment of neurotransmitter release and reception machinery. Using large-scale genetic screens in Drosophila we are uncovering the molecular mechanisms that form and maintain the active zone/receptor cluster dyad.