
Michael Wong, MD, PhD
Allen P. and Josephine B. Green Professor of Pediatric Neurology, WashU Neurology
- Phone: 314-362-8713
- Email: wong_m@nospam.wustl.edu
Cellular mechanisms of epileptogenesis and seizure-induced brain injury
The primary goal of my research laboratory is to understand biological mechanisms in the brain underlying epilepsy, with the ultimate purpose of developing new therapies for epilepsy patients. We have focused on investigating cellular and molecular mechanisms of epileptogenesis and seizure-induced brain injury in animal models of epilepsy. Both pharmacological and genetic models of epilepsy are studied on the behavioral, circuit, cellular, and molecular level. A wide variety of experimental approaches are utilized, including electrophysiological (from patch-clamping in culture cells and slice preparations to in vivo video-EEG monitoring), histological (conventional and fluorescent assays), molecular biological (Western blotting, polymerase chain reaction), and modern cellular imaging (confocal and two-photon microscopy of in vivo, live slice, and fixed tissue preparations) techniques.
Epileptogenesis refers to abnormal biological processes in the brain that cause the development of epilepsy. As many patients with epilepsy are intractable to current treatments, understanding the cellular and molecular mechanisms of epileptogenesis may lead to novel, more effective therapies for epilepsy. To investigate mechanisms of epileptogenesis, we have used pharmacological seizure models to identify developmental differences in neurotransmitter systems that contribute to decreased seizure threshold in immature animals. In several genetic models of epilepsy, we are investigating the molecular mechanisms involved in epileptogenesis due to specific genetic mutations. A major recent focus of the lab has been on an animal model of the human disease, Tuberous Sclerosis Complex (TSC), which is one of the most common genetic causes of epilepsy. We have characterized the epilepsy in this mouse model and described a number of cellular and molecular abnormalities in glia and neurons that contribute to epileptogenesis in the mice, such as astrocyte proliferation, neuronal death, impaired glial buffering of glutamate and potassium, and dysregulation of specific cell signaling pathways. Notably, we showed that pharmacological inhibition of one of these signaling pathways (the mTOR pathway) completely prevented the development of epilepsy and the underlying brain abnormalities in these mice. Recently we have shown that mTOR inhibition may also have anti-epileptogenic actions in models of acquired epilepsy due to brain injury. Ongoing studies should continue to define other mechanisms of epileptogenesis and identify novel therapeutic targets in various models of epilepsy.
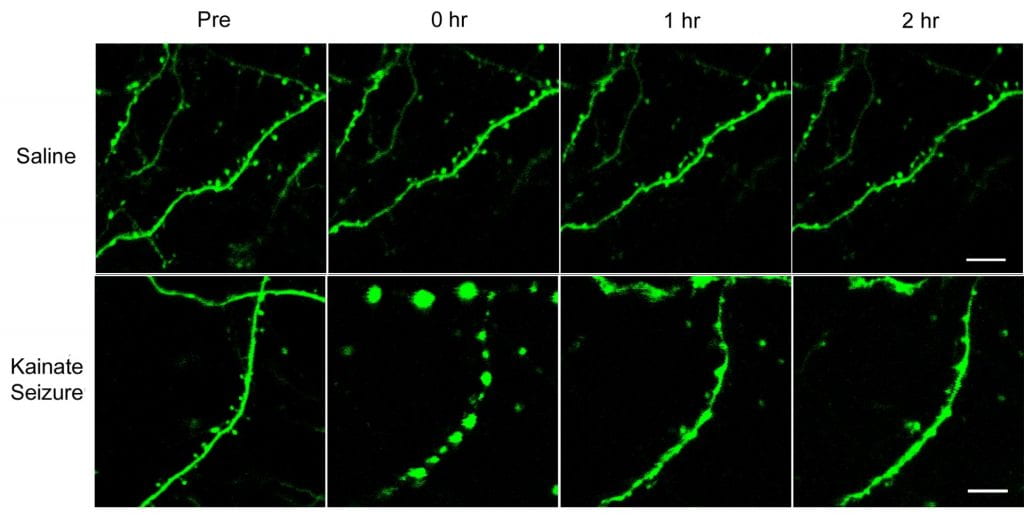
The second major area of research involves mechanisms of seizure-induced brain injury. Seizure-induced brain injury, including both lethal (neuronal cell death) and “non-lethal” mechanisms of cellular injury, could account for cognitive and other neurological deficits that often develop in epilepsy patients. We have studied various pharmacological models of non-convulsive status epilepticus and compared differences in seizure-induced neuronal death in these models, as assayed by conventional histological and novel fluorescent markers of cell death. Recently we have utilized modern time-lapse multiphoton imaging methods to directly visualize acute, dramatic structural changes in dendrites and dendritic spines in living mice during seizures in vivo. We have correspondingly found that seizures can activate specific intracellular signaling pathways that lead to breakdown of the normal actin cytoskeleton of dendrites resulting in this “non-lethal” dendritic damage. As dendritic spines normally function in synaptic plasticity and learning, this seizure-induced dendritic injury may represent a mechanistic basis for cognitive dysfunction in epilepsy patients. We have also found that pharmacological stabilization of actin is effective in reducing seizure-induced dendritic injury in mice, thus pointing to a novel therapeutic approach for treating the neurological consequences of epilepsy. Future studies will continue to dissect the mechanisms of seizure-induced brain injury and identify sites for potential therapeutic intervention.