Jeffrey Milbrandt, MD, PhD
James S. McDonnell Professor, WashU Genetics
- Phone: 314-362-4652
- Email: jmilbrandt@nospam.wustl.edu
Axonal degeneration, regulation of myelination, neuronal energetics and mitochondrial function in neuropathy and neurodegenerative disease
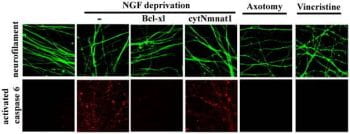
Axonal degeneration is a major component of many neurodegenerative diseases, including Alzheimer’s disease, amyotrophic lateral sclerosis (ALS), Parkinson’s disease, and multiple sclerosis. Mitochondrial dysfunction caused by mutations or toxins is also closely associated with both axonopathy and neurodegenerative disease. Through studies of the Wld mouse mutant, we found that enzymes involved in cellular energetics (NAD biosynthesis), the longevity-associated protein SIRT1, and resveratrol, a polyphenol found in red wine, can protect against axonal degeneration after injury or mitochondrial damage. To understand how deficits in neuronal metabolism lead to inappropriate responses to stress and result in disease, we are exploring how changes in energy utilization and production influence neuronal function in mice engineered to have deficits in NAD biosynthetic pathways, mitochondrial proteins, and AMP kinase subunits.
We identified a number of growth factors that make up the GFL family of neurotrophic factors (GDNF, neurturin, artemin, and persephin). These proteins activate the Ret receptor tyrosine kinase, which is mutated in many human diseases including Multiple Endocrine Neoplasia type 2 (MEN2), Hirschsprung’s disease, and thyroid papillary carcinoma. These neurotrophic factors regulate proliferation of neuronal stem cell-like precursors. They also promote survival and development of multiple neuronal populations including motor and dopaminergic neurons, neurons that degenerate in ALS and Parkinson’s disease. With this in mind, neurturin is now being tested in clinical trials as a treatment for Parkinson’s disease. We are using expression profiling, comparative genomics, and characterization of mice expressing Ret mutants that lack individual phosphorylated tyrosine residues to understand the role of Ret in disease.
Defects in peripheral nerve regeneration and myelination due to either traumatic injury, exposure to toxins, or from acquired (e.g. diabetes) or hereditary (e.g. CMT) disease cause significant morbidity. We found that Schwann cell myelination is controlled by Egr2/Nab protein transcription complexes. We are studying the function of these proteins using mouse models of CMT in attempts to identify the network of genes that is activated in Schwann cells when they myelinate axons. In addition, we have identified a large number of transcripts that are induced in Schwann cells surrounding injured axons that are likely to be involved in myelination and nerve regeneration. In vitro myelination assays using lentiviral vectors and siRNA constructs are being used to examine the function of these candidates.