
Circadian neurobiology and developmental neurobiology
My laboratory studies questions in two subjects using the model system Drosophila – circadian neurobiology and developmental neurobiology.
The behavioral studies focus on the ~150 neurons that constitute the neural circuitry underlying the circadian organization of locomotion. Our approach defines transmitter phenotypes expressed by circadian pacemaker neurons. We then analyze cell interactions by pharmacology, behavoral genetics and imaging. We have shown that the neuropeptide PDF represents the principal transmitter released by a subset of the critical pacemaker neurons in the fly brain. To pursue questions regarding the peptidergic organization of behavior, we identified the receptor for PDF and have mapped PDF receptor in the brain in two complimentary ways, using expression and activation methods. We are currently interested in defining the signaling pathways by which PDF synchronizes the molecualr oscillator in other pacemaker neurons. Also we are using realtime measurements of pacemaker activity to better understand the contributions and interactions of different circadian clock neurons.
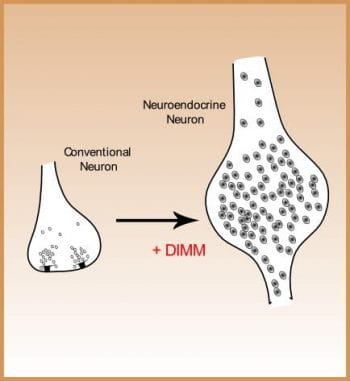
The developmental studies address the genetic programs underlying neuron diversification. In particular, we study how peptidergic neurons acquire their general and specific properties. Specific properties include the selection of a unique neuropeptide phenotype. General properties include expression of a robust secretory machinery. We have defined a transcriptional control mechanism in Drosophila headed by the bHLH protein DIMM that operates in diverse neurosecretory neurons and which is responsible for the cells’ ability to accumulate process, and package large amoutns of secretory peptides and peptide hormones. This transcription factor confers these properties onto non-peptidergic neurons and directly controls terminal differnetiation. We would like to understand how this transcriptional program underlies the cell biology of secretory cells in detail. To do this, we identify DIMM molecular targets and study their individual roles. In addition, we have initiated parallel studies in mouse where an orthologous factor is expressed by neurosecretory neurons of the brain.
Research Group Affiliations
- Co-Organizer, Clocks & Sleep Club
- Neurogenetics & Transcriptomics