Yuna Ayala, PhD
Associate Professor and Vice Chair, SLU Biochemistry & Molecular Biology
- Phone: 314-977-9247
- Email: yayala@nospam.slu.edu
Role of RNA processing and TDP-43 function in neurodegeneration
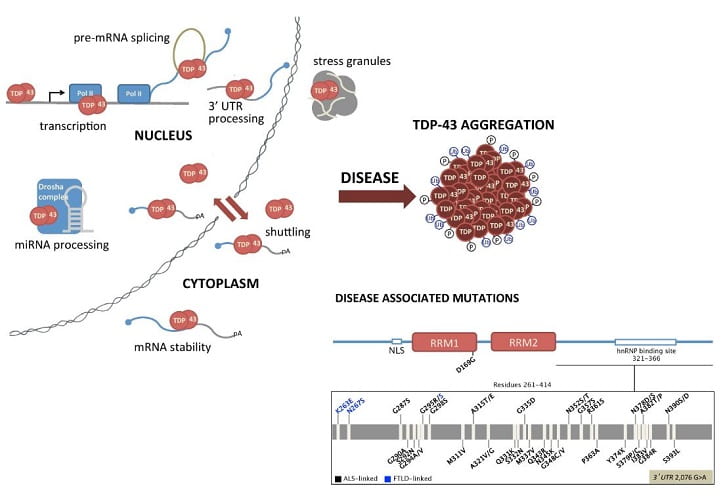
My group seeks to understand how defects in RNA processing lead to human disease. In particular, we are interested in the elucidation of TDP-43 function and its connection to the development and progression of neurodegenerative disorders. TDP-43 is an RNA and DNA binding protein involved in the regulation of various RNA-associated mechanisms. TDP-43 is the main component of inclusion bodies in different types of neurodegeneration, but primarily in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). Various lines of evidence, such as the identification of more than 40 TDP-43 mutations in ALS and FTLD patients, strongly suggest that TDP-43 dysfunction contributes to neurodegeneration. Despite the significant recent progress in the elucidation of TDP-43 function, the related processes that play a role in pathogenesis remain unknown. Our goals are to determine cellular pathways that regulate TDP-43, to define models of TDP-43-mediated RNA processing, and to understand their connection to disease.