Min Kyung Shinn, Rohit Pappu investigate nuclear speckles
Microphases are targets for therapeutic intervention for neurodegenerative disorders


Min Kyung Shinn, Rohit Pappu investigate nuclear speckles

Every function in a cell is associated with a particular protein or group of proteins, typically in a well-defined three-dimensional structure. However, intrinsically disordered regions of proteins defy this structure-function paradigm. A team of researchers in the McKelvey School of Engineering at Washington University in St. Louis has developed an algorithm to understand how intrinsically […]

Using advanced techniques in biophysical chemistry, a team led by Meredith Jackrel, PhD, an associate professor of chemistry, has achieved unprecedented views of a protein that may play a pivotal role in some cases of amyotrophic lateral sclerosis (ALS) and the related disorder frontotemporal dementia (FTD). Their work could open doors to new approaches for treatment […]
Scopus list of Hope Center faculty members’ publications for the week of June 8, 2025

Scientists from St. Jude Children’s Research Hospital and Washington University in St. Louis report mechanistic insights into the role of biomolecular condensation in the development of neurodegenerative disease. The collaborative research, published in Molecular Cell, focused on the interactions that drive the formation of condensates versus the formation of amyloid fibrils and how these relate to […]

Biomolecular condensates are distinct molecular communities made of DNA, RNA and proteins that “condense” molecules to key locations inside cells. Intense efforts have focused on uncovering the numerous ways in which condensation is controlled, modulated and regulated inside cells. In research published May 23 in Science Advances, biomedical scientists at Washington University in St. Louis and […]

Biomolecular condensates are shifting blobs in our cells that organize cellular matter. They are distinct molecular communities made of DNA, RNA and proteins that “condense” molecules to key locations, yet they frequently defy description. Partly this is because they are so small, they cannot be measured using traditional microscopes. “These blobs were once described as […]
Scopus list of Hope Center faculty members’ publications for the week of March 9, 2025

On behalf of the Medical Scientist Training Program Committee, we are pleased to announce the recipients of this year’s Spencer T. and Ann W. Olin Medical Science Fellows: The Olin Fellowships recognize superior accomplishments in biomedical research by doctoral students at Washington University. 32 outstanding students were nominated for the Olin Fellows Award this year, […]

Rohit V. Pappu, PhD, the Gene K. Beare Distinguished Professor of Biomedical Engineering and director of the Center for Biomolecular Condensates at Washington University in St. Louis, has been selected as an American Physical Society Fellow. Pappu was selected for his innovative and fundamental studies regarding intrinsically disordered proteins and phase transitioning behaviors using polymer […]

Scientists at the McKelvey School of Engineering at Washington University in St. Louis sort the rules governing putty-like biomolecular condensates.
Scopus list of Hope Center faculty members publications for the week of June 2, 2024

Scopus list of publications for the week of April 14, 2024
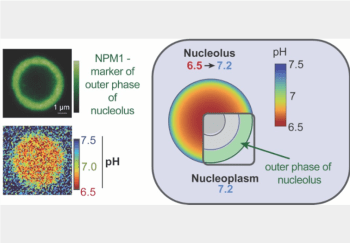
Scientists trying to understand the physical and chemical properties that govern biomolecular condensates now have a crucial way to measure pH and other emergent properties of these enigmatic, albeit important cellular compartments. Condensates are communities of proteins and nucleic acids. They lack a membrane and come together and fall apart as needed. The nucleolus is […]

Rohit Pappu, collaborators from Duke University make discovery with model fungus
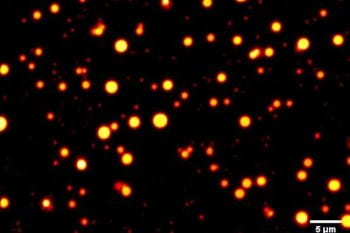
Rohit Pappu, collaborators find networking afforded by interactions among RNA molecules can enable different phase behavior when heating or cooling
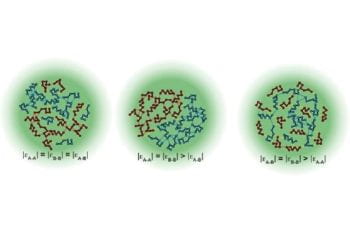
WashU and St. Jude groups uncover the rules for organization of cellular condensates implicated in ALS

Rohit V. Pappu, PhD, an internationally renowned researcher in biomolecular condensates and intrinsically disordered proteins, was installed Oct. 9 as the Gene K. Beare Distinguished Professor of Biomedical Engineering at Washington University in St. Louis. Pappu is a professor of biomedical engineering and director of the Center for Biomolecular Condensates at the McKelvey School of […]
Scopus list of publications for September 17, 2023
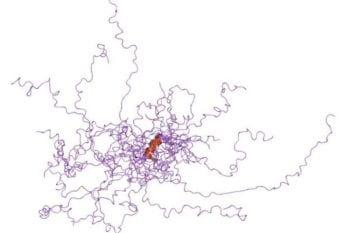
Intrinsically disordered proteins (IDPs) are defined by structural diversity, and the determinants of this diversity are an important area of biophysical investigation. IDPs are involved in a range of important biological processes, including cell signaling and regulation, that allow healthy cells to respond to environmental factors appropriately, but they are also associated with human diseases […]
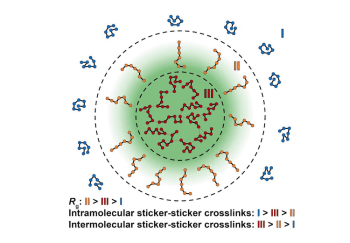
Research from labs of Rohit Pappu, collaborators sheds light on condensate characteristics
Symposium brings together key leaders in the field
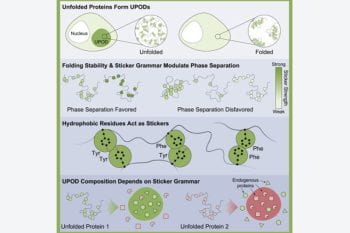
Research uncovers how unfolded proteins are discarded, and how a perfectly good protein can wind up in the trash

Scopus list of publications for September 4, 2022
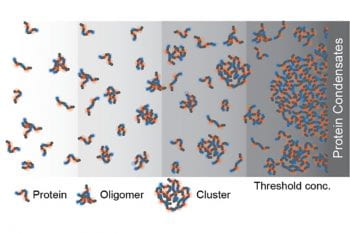
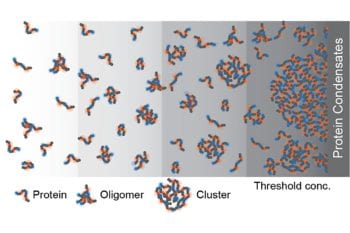
Every cell contains millions of protein molecules. Some of them have the ability to phase-separate to form non-membrane-bound compartments, called biomolecular condensates, inside a cell. It has long been assumed that there was no further structure underlying these condensates, only solution-soluble proteins. A research group led by Rohit Pappu, PhD, the Gene K. Beare Distinguished Professor […]
Intrinsically disordered regions (IDRs) of proteins, when tethered to folded domains, function either as flexible tails or as linkers between domains. Most IDRs are composed of a mixture of oppositely charged residues. Recent measurements of tethered polyampholytes have shown that arginine- and lysine-rich sequences tend to behave very differently from one another. In a paper […]

Longstanding collaboration leads to new findings about processes associated with neurodegenerative diseases

These vesicles are critical for proper cell function

List of publications for the week of November 8, 2021

NIH awards $3.1 million grant for Washington University, St. Jude ALS research