
Meredith Jackrel, PhD
Associate Professor, WashU Chemistry
- Phone: 314-935-6530
- Email: mjackrel@nospam.wustl.edu
Engineering and applying protein-remodeling factors to counter neurodegenerative disease
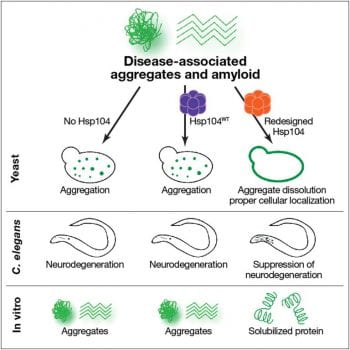
We study protein folding and misfolding, and how protein misfolding can lead to neurodegenerative disease. We are particularly interested in protein disaggregases, a type of protein-remodeling factor that can dissolve aggregated proteins and restore the misfolded species to native fold and function. We have found that a protein from yeast, Hsp104, that naturally regulates yeast prions can also disassemble disordered aggregates, pre-amyloid oligomers, and amyloid fibrils comprised of proteins implicated in human disease, though the activity of Hsp104 against these non-native substrates is diminished. Therefore we aim to apply protein engineering and directed evolution approaches to enhance and tune the activity of Hsp104 and other protein-remodeling factors. Variants we have developed suppress the aggregation, toxicity, and mislocalization of diverse proteins that aggregate in ALS and Parkinson’s disease. We have also demonstrated that these proteins eliminate preformed amyloid fibrils and disordered aggregates and also suppress dopaminergic neurodegeneration in an animal model of Parkinson’s disease. We are now eager to further fine-tune the specificity of these variants and apply these agents as tools to investigate the underpinnings of neurodegenerative disease. We also aim to develop methods to boost the activity of other protein-remodeling factors.